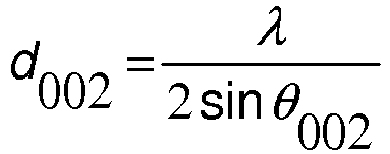This activity is planned for the third and fourth classes of the first two months with the intention of building with the students the concept of electric current in a course of Electromagnetism in High School. It was inserted after the students worked with a simple circuit consisting of a battery, one or two wires and a lantern lamp. In this class the students set up the circuit and had to explain how and why the light comes on, using sentences and drawings, with the wire extended so that the teacher could perceive the student’s ideas about the metal structure and how they imagined something “moving”. in this structure. The graphite powder for li-ion battery anode use is essential now.
How to intervene with spontaneous conceptions
From the answers given by the students about circuits I decided to insert the experiments “electrolysis” and “Daniell’s cell” next, with the intention of building with them the microscopic model, that is, I intend to go from macroscopic to microscopic.
Specific activity for students to rework their conceptions
Electrolysis allows students to notice things that are visible, for example, bubbles rising in the graphite, smell of “candida” (chlorine). This experience helps students broaden their explanations of electrical current, helps convince students that something is circulating in both the wire and the solution and that the circuit is closed in the solution due to the movement of “things” (ions, charges). ), students may conclude that the circuit is closed because the led lights up and the motor runs.
Objectives
The purpose of this experiment is to understand electrical current in a liquid medium, to help students improve their explanations, and through observation, to convince them that something circulates in the circuit and that in liquid the circuit is closed with the movement of something (ions), charges.
Contents
The content to be explored with this activity is the idea, the concept of electrical current in the wire and liquid, ionization and chemical reactions.
Conceptual Expectations
This experiment makes it possible to conflict with some of the students’ ideas, for example, the idea that positive energy comes out of one side of the battery and negative energy comes out of the battery and the lamp is lit.
What happens in practice
During the activity the teacher can dialogue with the students by asking the following questions:
- What is the constitution of water? What is she formed of? When they put the salt in the water, did anything change?” What?
- What constitutes salt?
- What’s in the liquid now?
- Think and find out what salt is made of.
The following answers are expected during the experiment:
- Saltwater and graphite are conducive to energy.
- As the lamp was lit, the circuit is closed.
- Particles, electrons or charges pass through pencils and wire.
- We noticed that bubbles (gas) formed in the graffiti because a chemical reaction is taking place.
- We smell bleach.
If students give similar answers, the teacher can talk to the students and ask the following questions:
- Where does this energy come from?
- How does it originate?
- How do you keep up?
- Where are the particles? And the charges?
Materials Required
The setup of this experiment is quite simple, requiring: a plastic pot with two holes in the lid for pencil placement, two sharpened pencils at both ends, water, table salt (NaCl), 1.5 V batteries, a 9 V battery, connecting wires with alligators and one led.